
PPT Reactions in Aqueous Solutions PowerPoint Presentation, free download ID519535
the word equation is: potassium hydroxide + sulfuric acid → potassium sulfate + water; Key fact. Chemical equations contain an arrow and not an equals sign. The arrow means 'reacts to make'.

Sulphuric Acid 98, 24 Ton, Liquid, Karan Chemicals ID 22990740773
The concentration of this acid is known with greater accuracy. The data from this titration is then used to calculate a more accurate value for the concentration of the sodium hydroxide solution. A commonly used primary standard for titration with sodium hydroxide solution is the weak acid potassium hydrogen phthalate or KHP (\(\ce{C8H5O4K}\)).

Potassium Hydroxide & Sulfuric Acid Healthfully
2. Measure the temperature of the solution. 3. Add an equal volume of 1.0 molar solution of potassium hydroxide and stir. 4. Measure the temperature of the mixture. We have become familiar with acid/base neutralization reactions. The reaction between sulfuric acid and potassium hydroxide leads to the formation of salt and water.

Potassium Hydroxide Sulfuric Acid,hydrochloric acid,nitric acid,hydrogen peroxide,glacial
Solubility of Hydrogen in Potassium Hydroxide and Sulfuric Acid. Salting-out and Hydration. P. Ruetschi; and ; R. F. Amlie; Cite this: J. Phys. Chem. 1966, 70, 3, 718-723.. Antisolvent Precipitation of Potassium Bicarbonate from KHCO3 + H2O + Ethanol/2-Propanol Systems in the CO2 Capture Process. Industrial & Engineering Chemistry Research.
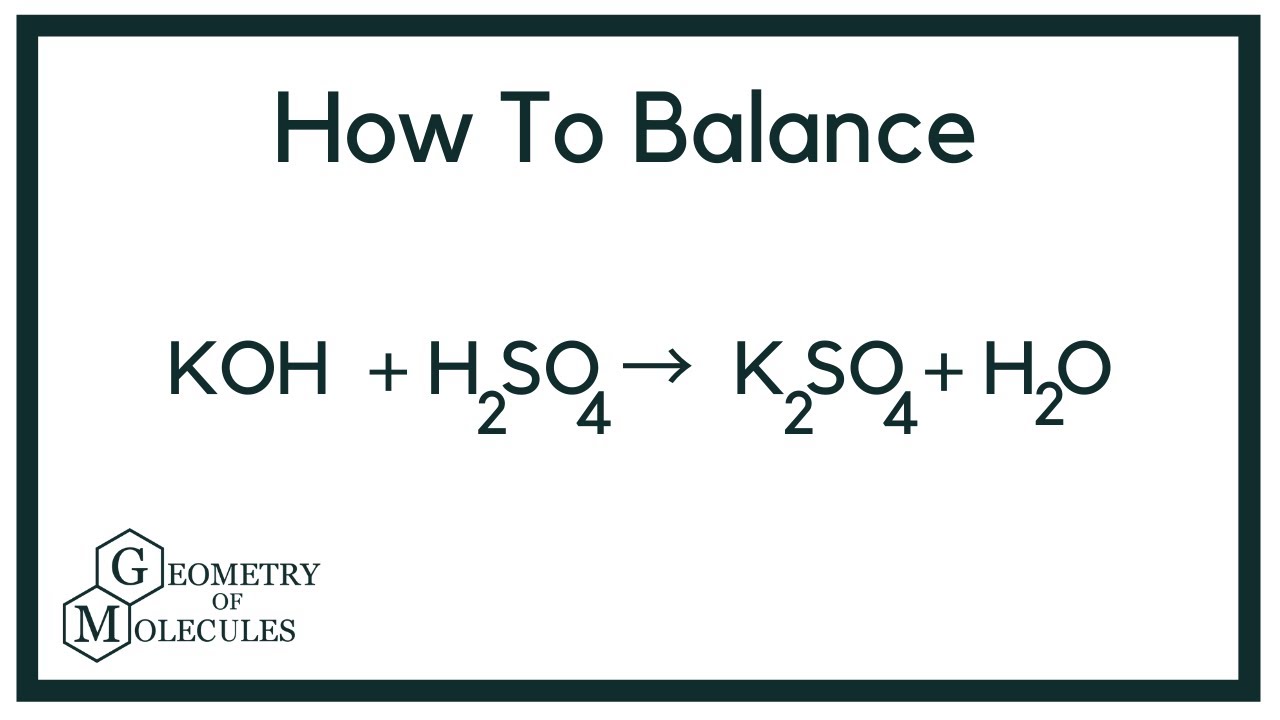
Balance KOH + H2SO4 = K2SO4 + H2O (Potassium Hydroxide and Sulfuric Acid) YouTube
Step-1:write the reaction. H₂SO₄ +KOH ----->K₂SO₄ +H₂O Step-2:In the left side, we have H SO₄ K O To balance this reaction means we need to equalize the number of these above atoms and polyatomic ion from both sides.
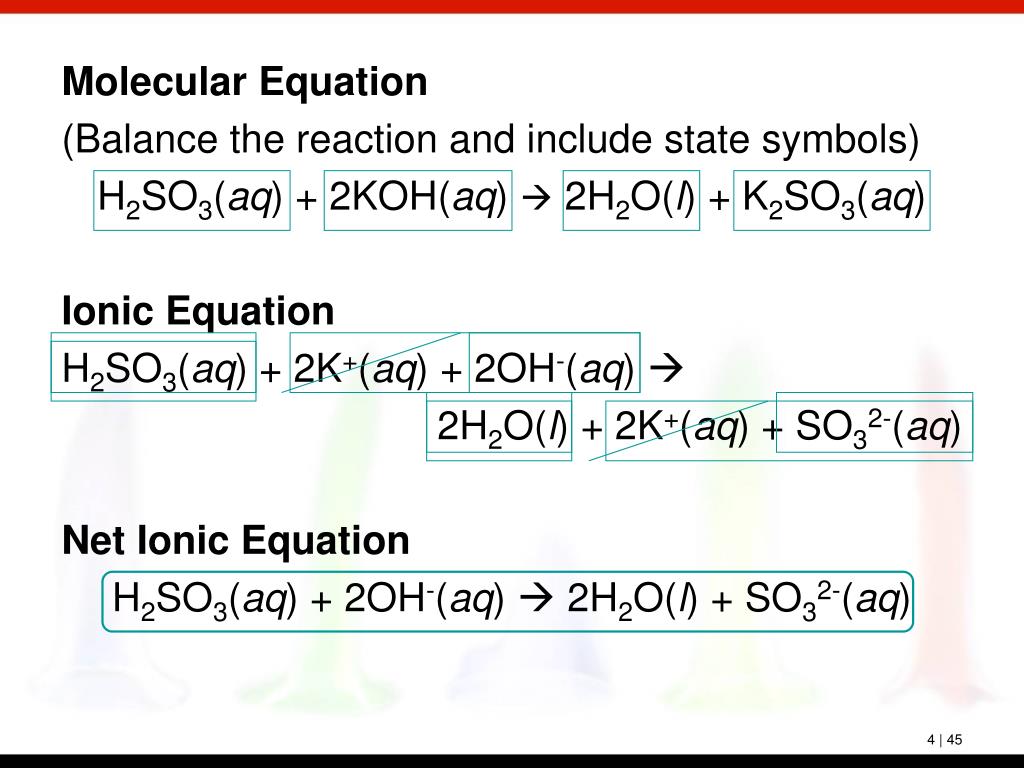
PPT Chapter 4 Chemical Reactions PowerPoint Presentation, free download ID5741189
Word Equation. Potassium Sulfate + Water = Potassium Hydroxide + Sulfuric Acid. K2SO4 + H2O = KOH + H2SO4 is a Double Displacement (Metathesis) reaction where one mole of aqueous Potassium Sulfate [K 2 SO 4] and two moles of liquid Water [H 2 O] react to form two moles of aqueous Potassium Hydroxide [KOH] and one mole of aqueous Sulfuric Acid [H 2 SO 4]

Balanced Equation For Sodium Hydroxide And Sulfuric Acid
For graded solutions of potassium hydroxide and of sulphuric acid, data from the International Critical Tables or more recent sources are used as the basis of tables giving the concentrations (wt.%) and densities corresponding to relative humidities in steps of 5 per cent. R.H. Sources of similar data for calcium chloride, sodium hydroxide.

Potassium Hydroxide & Sulfuric Acid
In this video we'll balance the equation KOH + H2SO4 = K2SO4 + H2O and provide the correct coefficients for each compound. To balance KOH + H2SO4 = K2SO4 +.
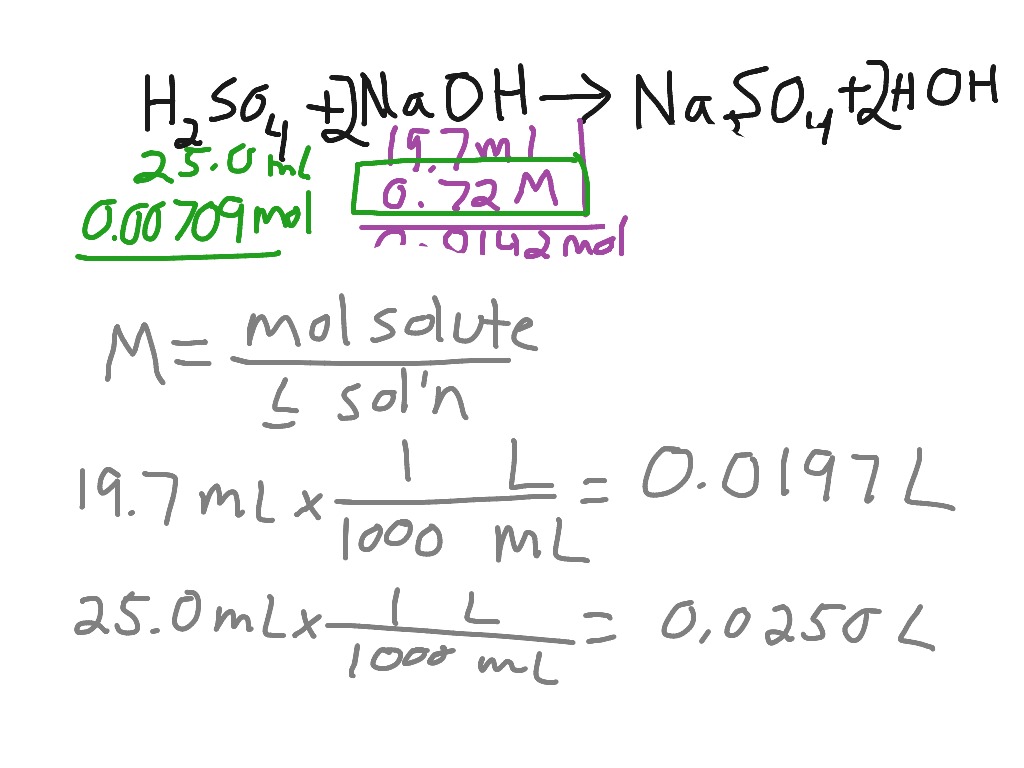
Titration of sulfuric acid with sodium hydroxide Chemistry, Acids and Bases, Stoichiometry
Summary A dilute acid has a sour or sharp taste Acids can be harmful and corrosive Common examples: vinegar, lemon juice, car batteries Bases and alkalis
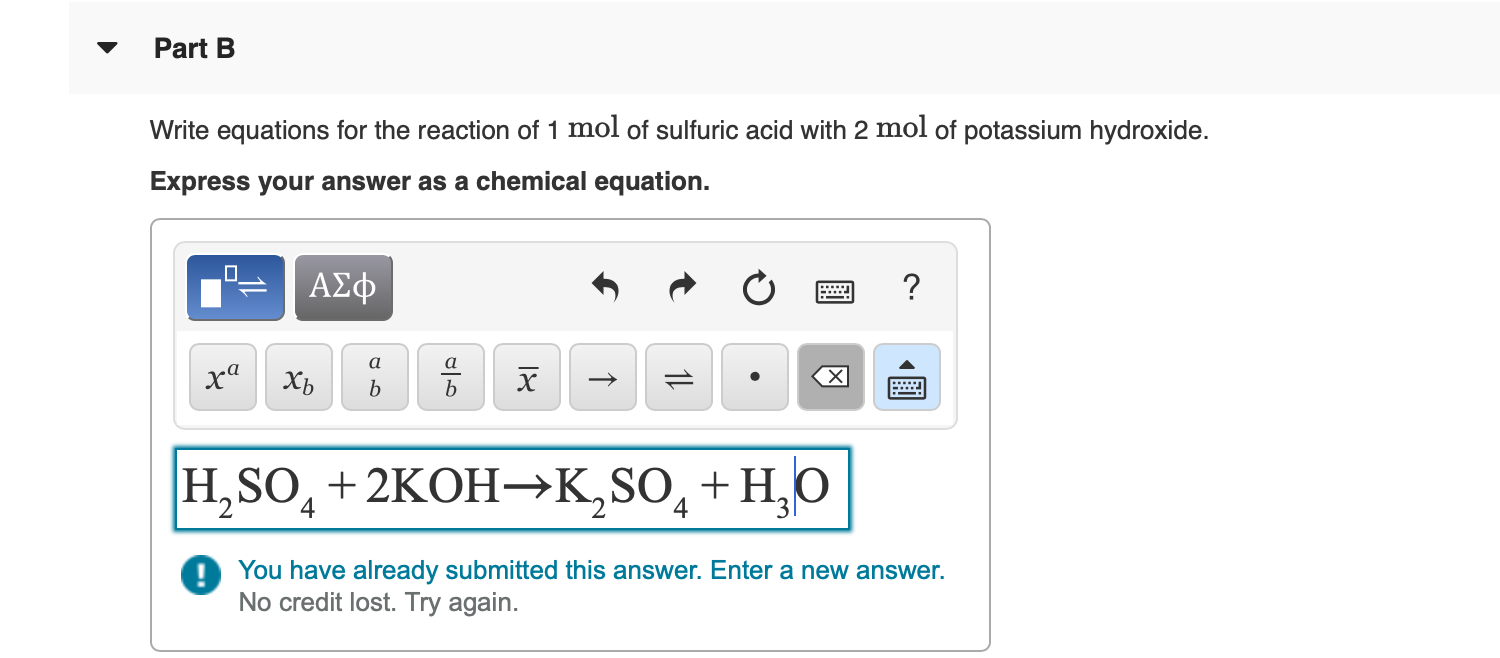
Answered Part B Write equations for the reaction… bartleby
Contact Like Us Sulfuric acid (H reacts with Potassium hydroxide (KOH) gives potassium sulfate (K) and water (H O) as products. Heat is released due to neutralization of a strong acid (H ) and a strong base (KOH). In this tutorial, we will discuss followings.

Reaction of sulphuric acid and potassium hydroxide Brainly.in
The ionization reaction of acetic acid is as follows: CH3CO2H(l) ⇌H2O(l) H+(aq) + CH3CO−2(aq) (4.7.4) Although acetic acid is very soluble in water, almost all of the acetic acid in solution exists in the form of neutral molecules (less than 1% dissociates), as we stated in section 4.1.

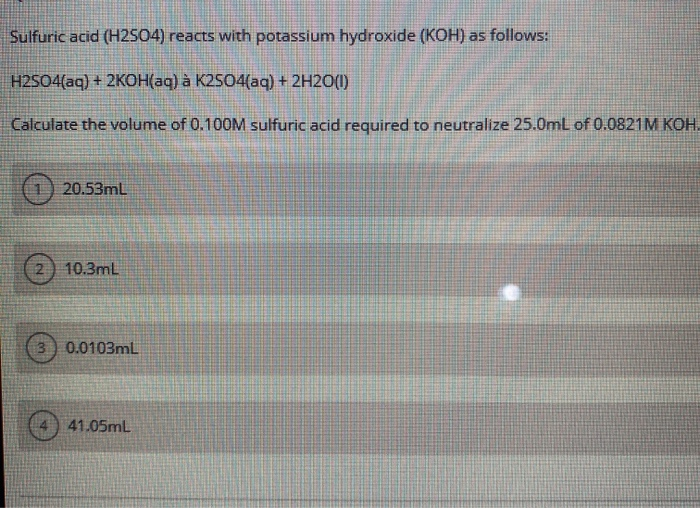
Solved Question 18 Sulfuric acid reacts with potassium
Sulfuric Acid and Potassium Hydroxide neutralize each other in the following reaction: H 2SO4 + 2KOH → K2SO4 +2H 2O In a neutralization reaction between an acid and a base the typical outcome is a salt formed by the positive ion from the base and the negative ion from the acid.
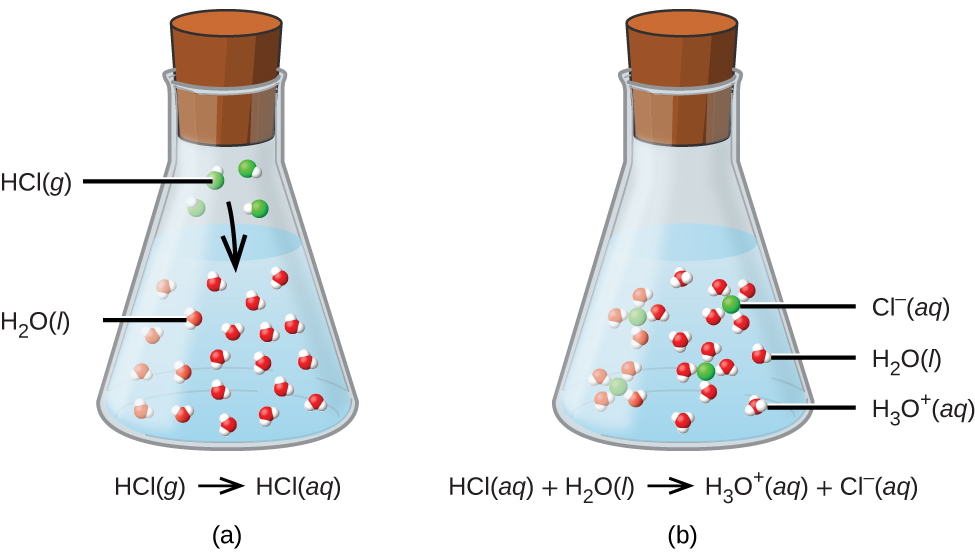
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UWMadison Chemistry 103/104
Acids and bases have another property: they react with each other to make water and an ionic compound called a salt. A salt, in chemistry, is any ionic compound made by combining an acid with a base. A reaction between an acid and a base is called a neutralization reaction and can be represented as: acid + base → H 2 O + salt.

Potassium Hydroxide and Sulphuric Acid ValentinojoysShannon
The reaction of sulfuric acid and potassium hydroxide produces an exothermic reaction, meaning it releases heat. When these two chemicals are mixed, the reaction forms a salt and water. The water created in this reaction can be either an acid or a base, depending on the concentration of the reactants. The heat of formation of potassium sulfate.
Solved Sulfuric acid (H2SO4) reacts with potassium hydroxide
Acids and bases that are completely ionized when dissolved in water are called strong acids and strong bases There are only a few strong acids and bases, and everyone should know their names and properties. These acids are often used in industry and everyday life. The concentrations of acids and bases are often expressed in terms of pH, and as an educated person, you should have the skill to.
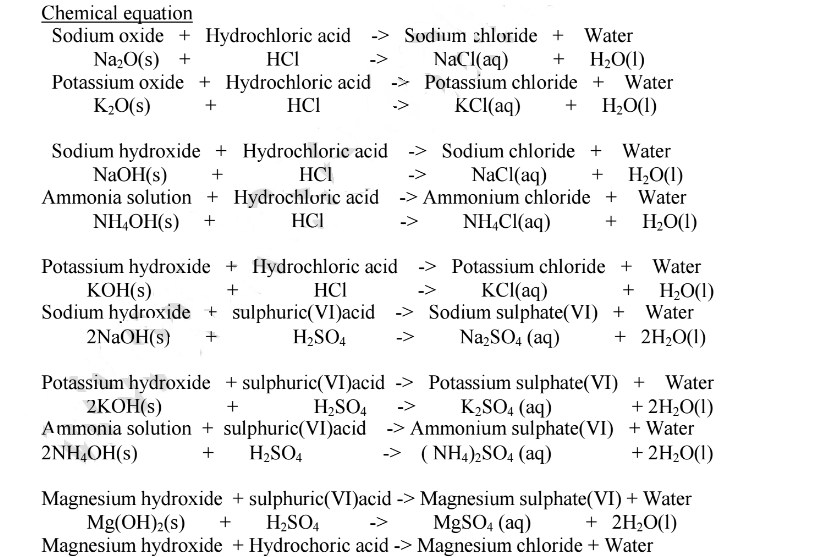
Chemistry Notes Acid, Bases and Indicators Revision Notes & Tests
What is the pOH when 5.0 L of a 0.45 M solution of sulfuric acid (H 2 SO 4) is titrated with 2.3 L of a 1.2 M lithium hydroxide (LiOH) solution? SOLUTION. To solve this problem we must first determine the moles of H + ions produced by the strong acid and the moles of OH-ions produced by the strong base, respectively: Acid: